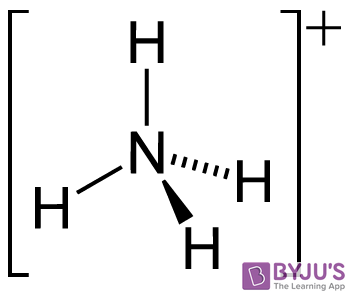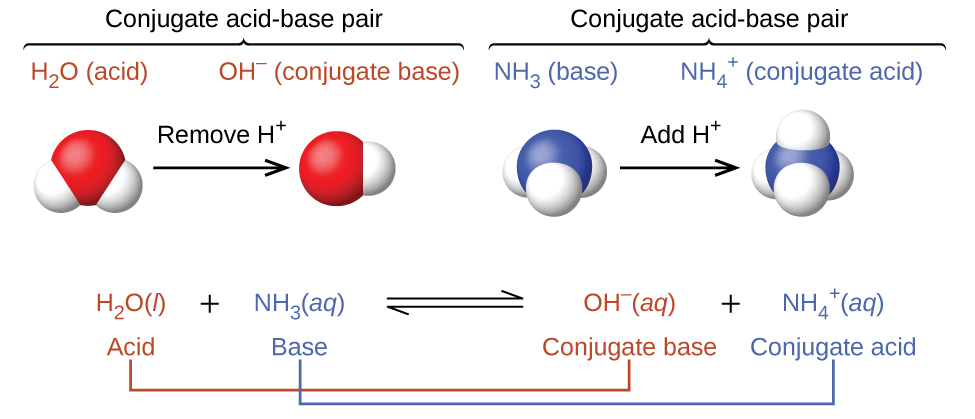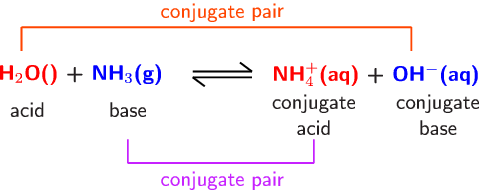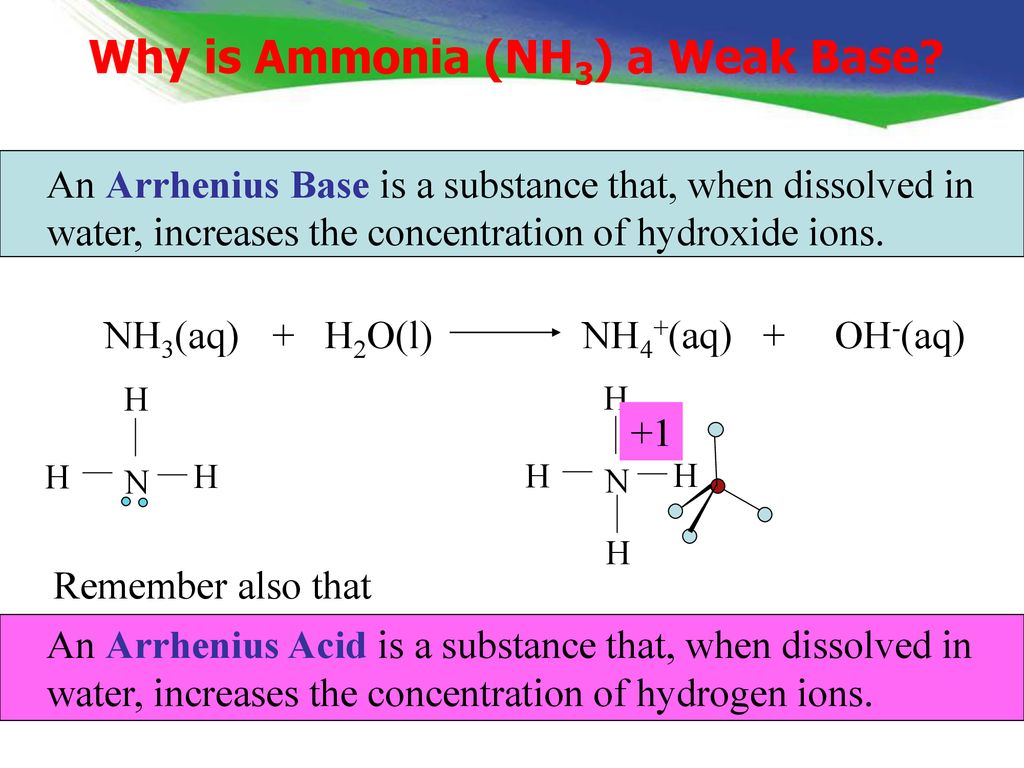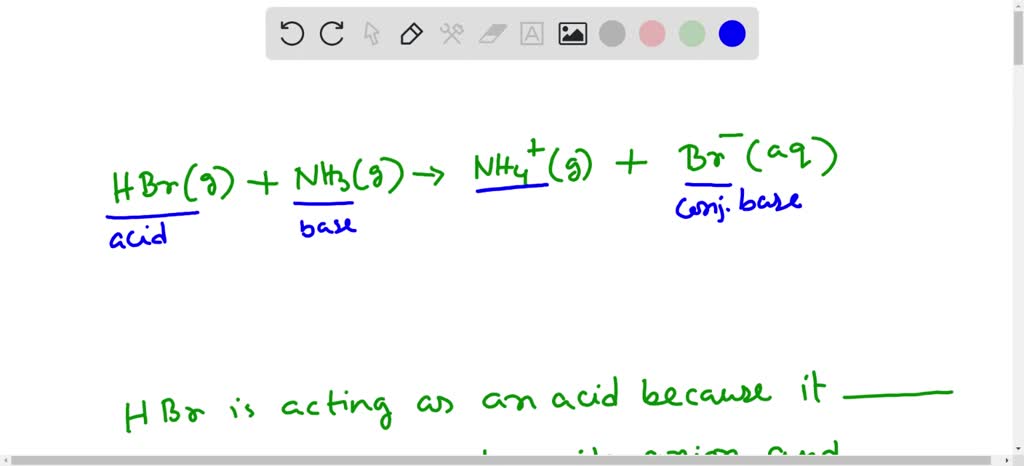
SOLVED: In the following acid-base reaction: HBr(g) + NH3(g) → NH4+(aq) + Br– (aq) HBr is acting as the acid, because it a proton to form bromide anion, and NH3 is acting
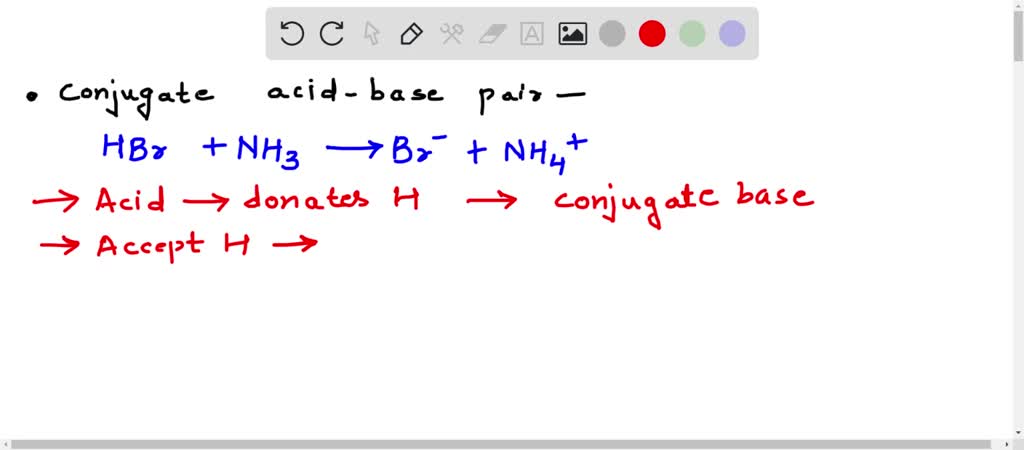
SOLVED: Identify the conjugate acid-base pairs in the reaction shown below HBr + NH3 Br + NH4 acid; chemPad Help Gianke conjugate base: chemPad Help Girekt base: chemPad Help Creke conjugate acid:

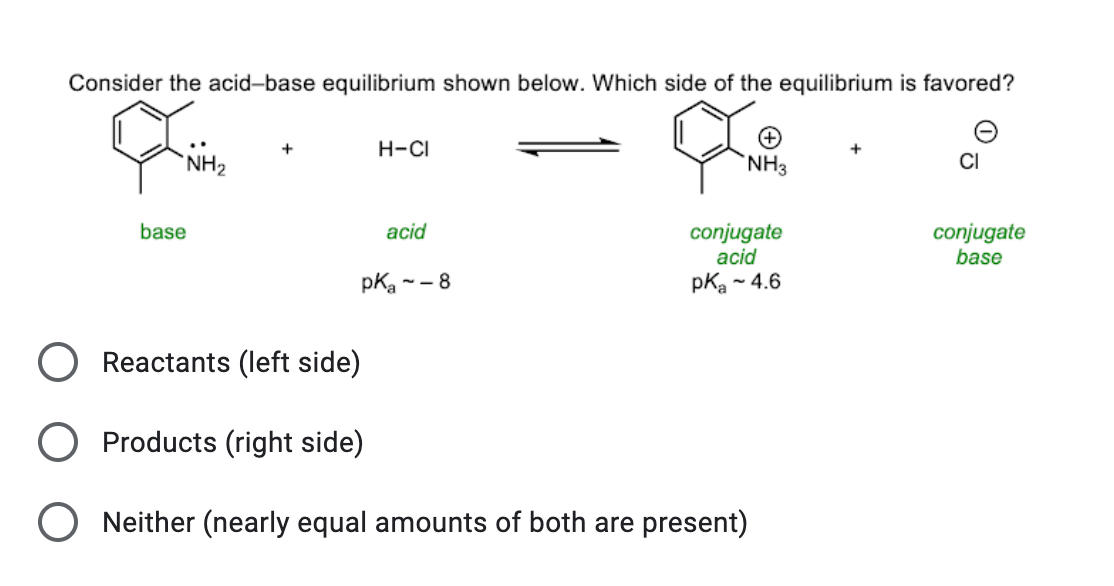
Electric Field-Driven Acid−Base Chemistry: Proton Transfer from Acid (HCl) to Base (NH3/H2O) | The Journal of Physical Chemistry A


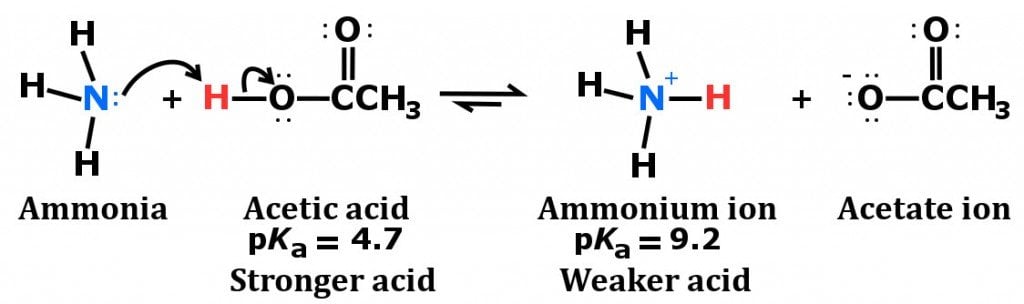



![Why does \\[N{H_3}\\] act as a Lewis base? Why does \\[N{H_3}\\] act as a Lewis base?](https://www.vedantu.com/question-sets/db470ea0-7477-412c-82d8-a4917a1132145281377342200076057.png)