![Cp*Rh(bpy)(H2O)]2+ as a coenzyme substitute in enzymatic oxidations catalyzed by Baeyer–Villiger monooxygenases - Chemical Communications (RSC Publishing) Cp*Rh(bpy)(H2O)]2+ as a coenzyme substitute in enzymatic oxidations catalyzed by Baeyer–Villiger monooxygenases - Chemical Communications (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/B504921K)
Cp*Rh(bpy)(H2O)]2+ as a coenzyme substitute in enzymatic oxidations catalyzed by Baeyer–Villiger monooxygenases - Chemical Communications (RSC Publishing)

SOLVED: When 1.053 g of tartaric acid (C4H6O6(s), MM = 150.1 g∙mol−1), is burned in a bomb calorimeter (Cbomb = 878 J∙ oC−1) that contains 968.6 g of water (Cp (H2O) =
![Cp∗Rh(bpy)(H2O)]2+: a versatile tool for efficient and non-enzymatic regeneration of nicotinamide and flavin coenzymes - ScienceDirect Cp∗Rh(bpy)(H2O)]2+: a versatile tool for efficient and non-enzymatic regeneration of nicotinamide and flavin coenzymes - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1381117702001649-gr1.gif)
Cp∗Rh(bpy)(H2O)]2+: a versatile tool for efficient and non-enzymatic regeneration of nicotinamide and flavin coenzymes - ScienceDirect

The System CaCl2–H2O: Thermodynamic Modeling and Flow Calorimetry Experiments at Elevated Temperatures and Pressures | Journal of Chemical & Engineering Data
![PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e9e458615e33c40d94b80a3189efa26d438c8a39/3-Table1-1.png)





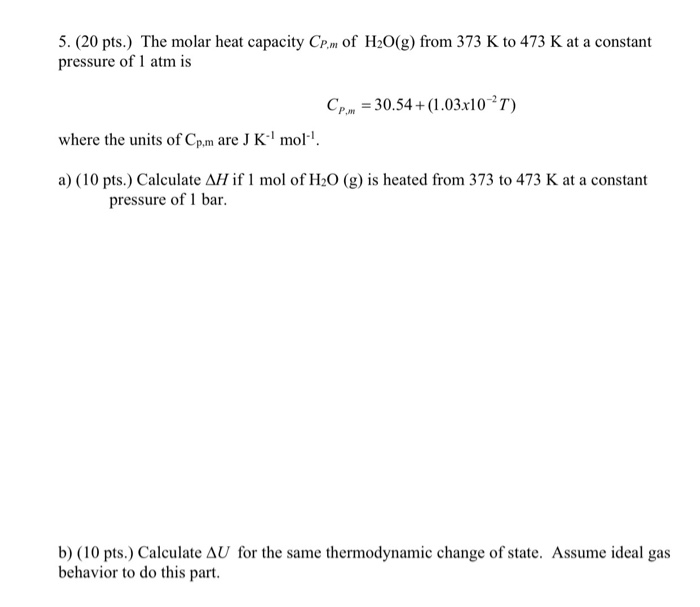

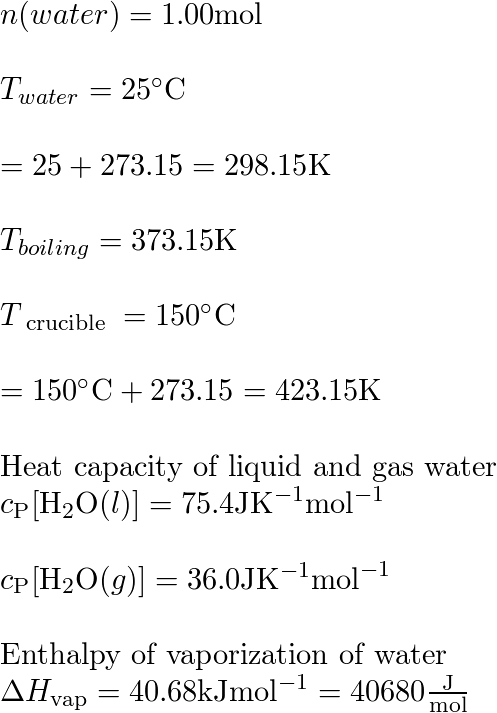


![Solved Heat Capacities pIH20l 37.65 J/K-mol Cp[H2O(l)]-75.29 | Chegg.com Solved Heat Capacities pIH20l 37.65 J/K-mol Cp[H2O(l)]-75.29 | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2F9c6%2F9c6322fb-d755-40a1-8987-9484af98164f%2FphpxcNGUz.png)