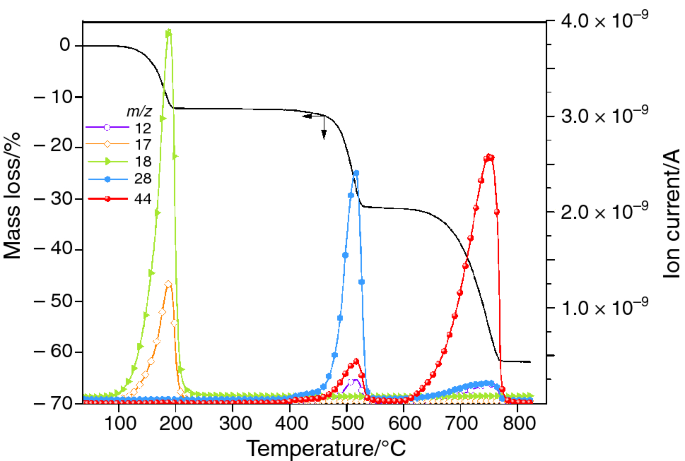
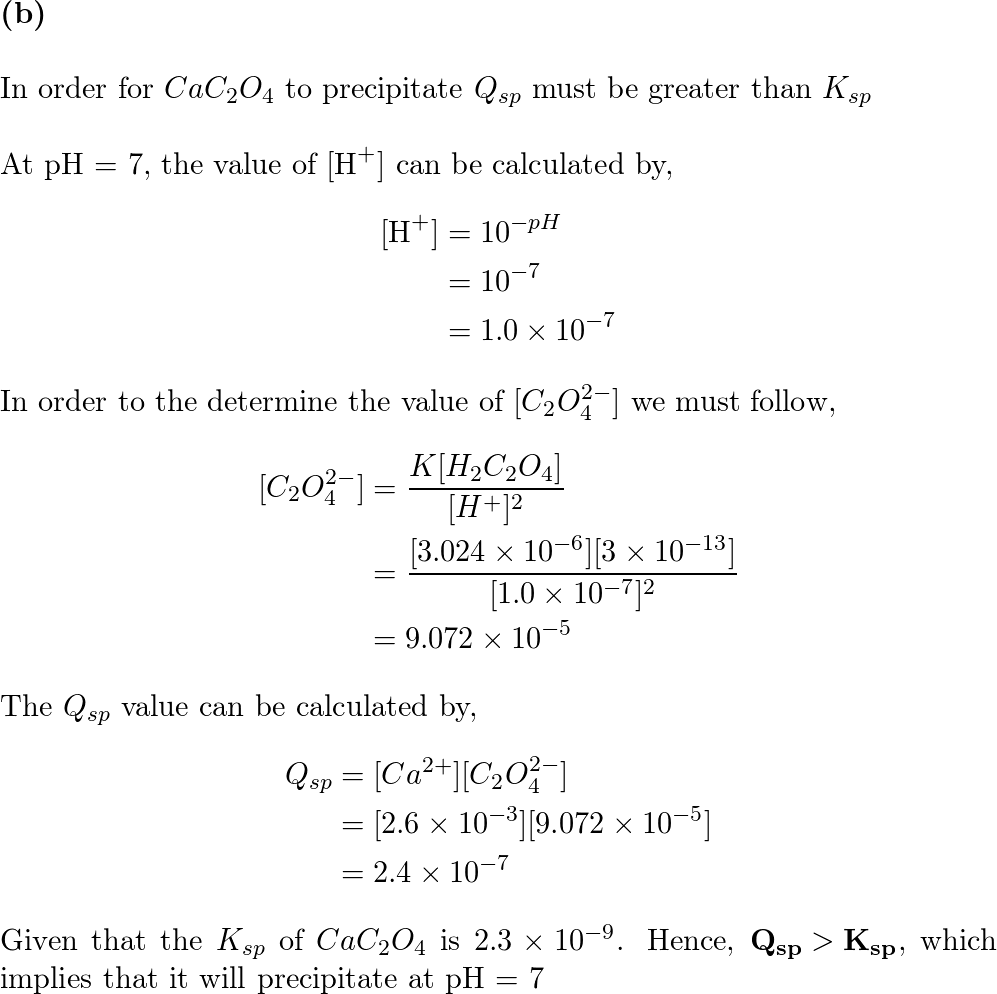Thermal Decomposition of CaC2O4·H2O Studied by Thermo-Raman Spectroscopy with TGA/DTA | Analytical Chemistry
Calcium oxalate, CaC2O4.H2O, is a sparingly soluble salt of analytical and physiological importance. - Sarthaks eConnect | Largest Online Education Community
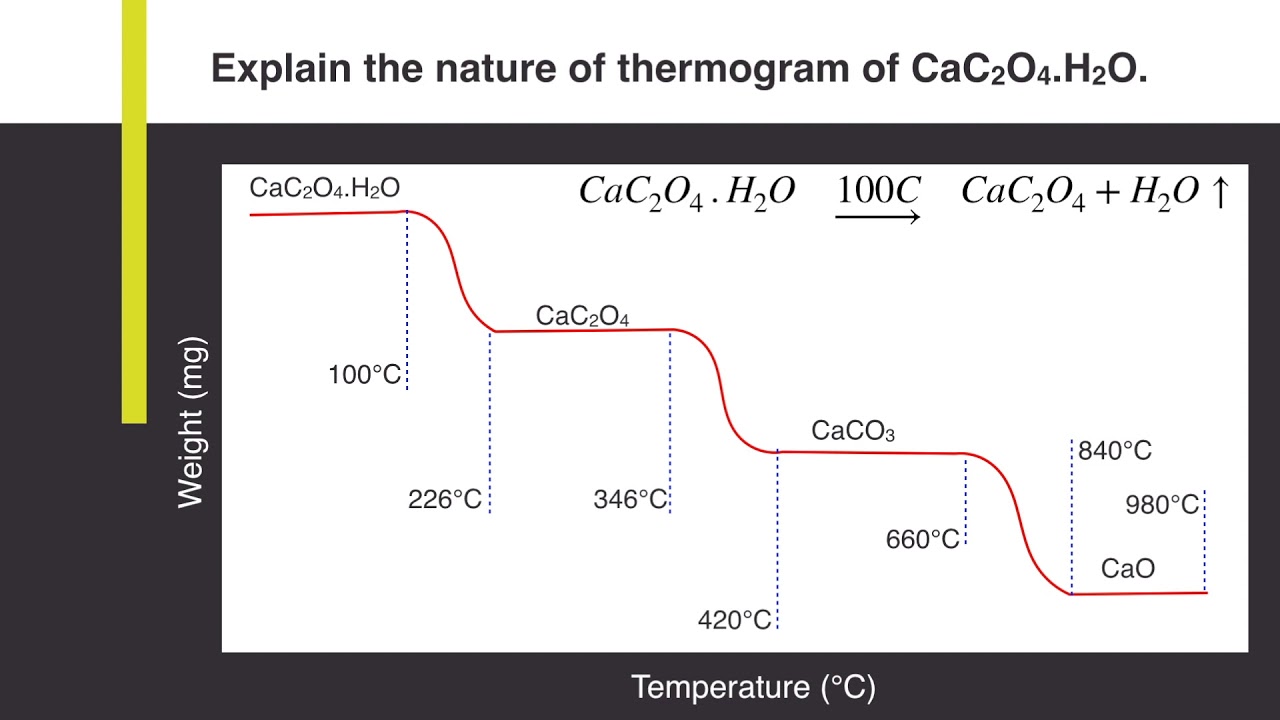
Explain the nature of thermogram of Calcium Oxalate Monohydrate (CaC2O4.H2O) | Analytical Chemistry - YouTube

Experiment 8 - Precipitation of CaC2O4*H2O from the Salt Mixture Unknown name Moon Stardust Limiting - Studocu

A 0.60 g sample consisting of only CaC2O4 and MgC2O4 is heated at 500 ^∘ C , converting the two salts of CaCO3 and MgCO3 . The sample then, weighs 0.465 g .

A 0.60 g sample consisting of only CaC2O4 and MgC2O4 is heated at 500 ^∘ C , converting the two salts of CaCO3 and MgCO3 . The sample then, weighs 0.465 g .
Thermal Stability of Calcium Oxalates from CO2 Sequestration for Storage Purposes: An In-Situ HT-XRPD and TGA Combined Study